3 Hydrogen 2 Carbon 1 Nitrogen Chemical Formula
2 x 149 298 ie 3 for N. X 2 1 2 moles of carbon.
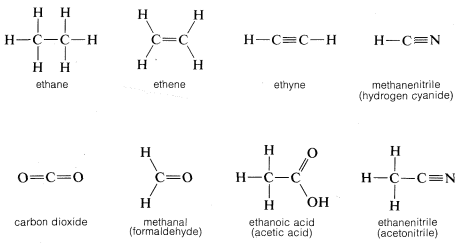
Compounds containing only carbon and hydrogen are called hydrocarbons.

. N E F w M w 5 4 1 0 8 2 Molecular formula C 6 H 8 N 2. Empirical weight 1 2 3 1 4 1 4 5 4 g. Given the molecular weight is 1 0 8 g.
Another sample of the same compound of mass 414 g yielded 260 g of SO 3 as the only sulfur containing product. 4-2 2 Cl. In a certain compound the ratio of C.
Y 35 2 7 moles of hydrogen. Get the answers you need now. 722 g Mg x 1 mol Mg243 g Mg 297 mol Mg for N.
The chemical formula is. The expression 2H 2 represents two molecules of diatomic hydrogen Figure 3. The molecular formula of the compound having two hydrogen atoms three oxygen atoms and one carbon atom is H 2 CO 3.
Chemistry 3-3 Hydrogen Nitrogen and Oxygen. A third sample of. C 2 Cl 3 F 3.
6-3 3 Cl. Carbon dioxide Chemical Formula. Empirical Formula C 3 H 4 N.
Thus molecular formula of the compound 2 empirical formula 2. 6-5 1 Cl. Nes global areas global regions.
Given the reaction 2a3bc2dδh1442 kj what will be the δh in kilojoules for the following reaction. It is made up of one carbon and two oxygen atoms. Pyridoxine contains 11 hydrogen atoms 8 carbon atoms 3 oxygen atoms and 1 nitrogen atom.
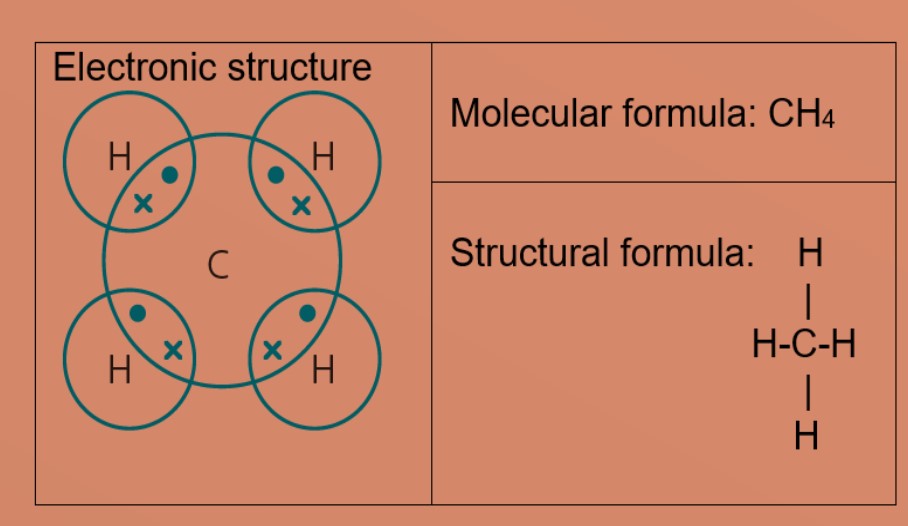
Jvtamez23 jvtamez23 11202020 Chemistry College answered Oxygen Hydrogen Nitrogen Carbon A. The formula is CH 4 and the name is methane. C 2 H 3 F 2 Cl.
3362 over 3304 1018 The oxygencarbon ratio is 1018 or approximately 1 and the hydrogencarbon ratio is approximately 2. O 53316 333. Carbon dioxide is a chemical compound composed of one carbon atom and two oxygen atoms.
1 Therefore the empirical formula is C 3 H 4 N. 2 5 3. Terms in this set 44 The force of attraction of gravity between 2 bodies is directly proportional to the.
H 671 67. Empirical formula mass 3 12 4 1 14 54. The chemical formula is.
Two moles of nitrogen for every one mole of nitrogen gas. Write the empirical formula and name the compound. A 252 g sample of a compound containing carbon hydrogen nitrogen oxygen and sulfur was burned in excess oxygen gas to yield 436 grams of CO 2 and 0892 grams of H 2 O as the only carbon and hydrogen products respectively.
C2H7N ethylamine or dimethylamine. Therefore the molecular formula for this compound is. 278 g N x 1 mol N140 g N 199 mol N 3 Divide by small.
C 40g12 g-mole 333. 2 x 100 200 and the formula of the compound is Mg 3 N 2. 12 90 102.
2 hydrogen 1 oxygen. This means that one mole of the original compound contained a total of. What are these areas calledclimate continents climate zo.
The world is divided into large areas based on their average temperatures and precipitation. Step 3 calculate the empirical formula weight. Express your answer as a chemical formula.
2 5 Integer ratio 0. CCl 2 F 2. 3304 over 3304 1000 H.
The chemical formula is. 653 over 3304 198 O. The simplest hydrocarbon contains one carbon atom and four hydrogen atoms.
Find an answer to your question a compound is 247 calcium 12 hydrogen 148 carbon and 593 oxygen. 1 carbon 4 hydrogen. 1 sulfur 2 oxygen.
It represents a diatomic molecule of hydrogen consisting of two atoms of the element that are chemically bonded together. 113 90 203. 142 90 232.
1 carbon 2 oxygen. It is a gas at room temperature. 297 mol l99 mol 149 for N.
N 1 2 9. H 2 is a molecular formula. D Which model represents the chemical formula NO2 nitrogen dioxide.
Express your answer as. 1 nitrogen 3 hydrogen. Z 05 2 1 mole of nitrogen.
199 mol l99 mol 100 4 Multiply til whole. If we continue adding carbon atoms and hydrogen atoms the formulas and names for the first ten hydrocarbons are. 2 5 1.
5 Molar ratio of C. Well begin with the compounds of carbon and hydrogen. What is the chemical formula for pyridoxine.
The expression 2H on the other hand indicates two separate hydrogen atoms that are not combined as a unit. Its formula is textCtextO_2 Carbon dioxide is a chemical compound that exists naturally in the atmosphere. What is the chemical formula for pyridoxine.
1 atom of carbon 1 atom of hydrogen and 3 atoms of oxygen Ccarbon Hhydrogen O3 oxygen What is the formula for a chemical compound that has 2 atoms of hydrogen 1 atom of carbon and 3 atoms of. C 333333 1. Oxygen Hydrogen Nitrogen Carbon A.
The correct answer is B. Step 2 calculate the atom ratio by dividing the mole by the lowest number of mole.
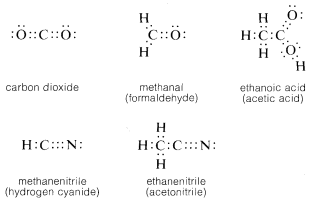
2 1 Structural Formulas Chemistry Libretexts
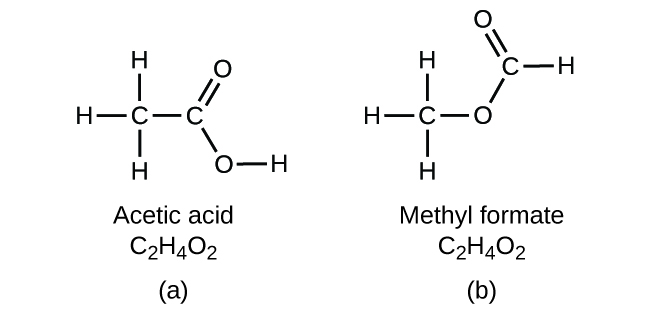
2 4 Chemical Formulas Chemistry
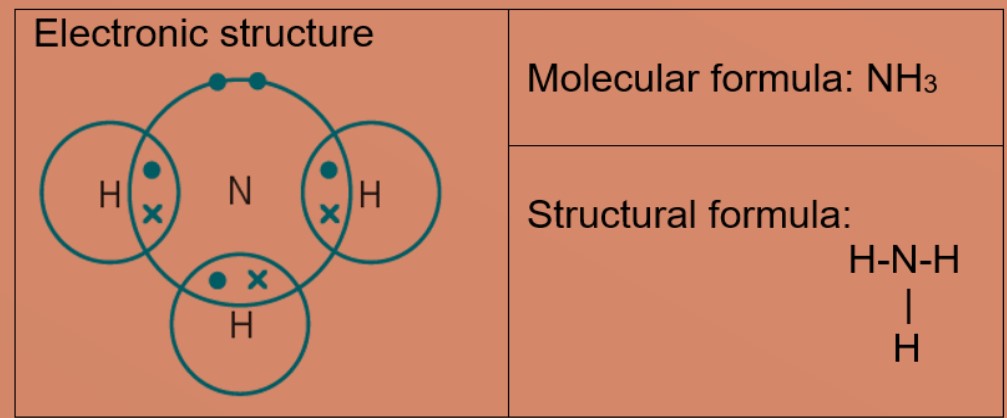
Covalent Bonding And Molecules

Organic Molecules Microbiology
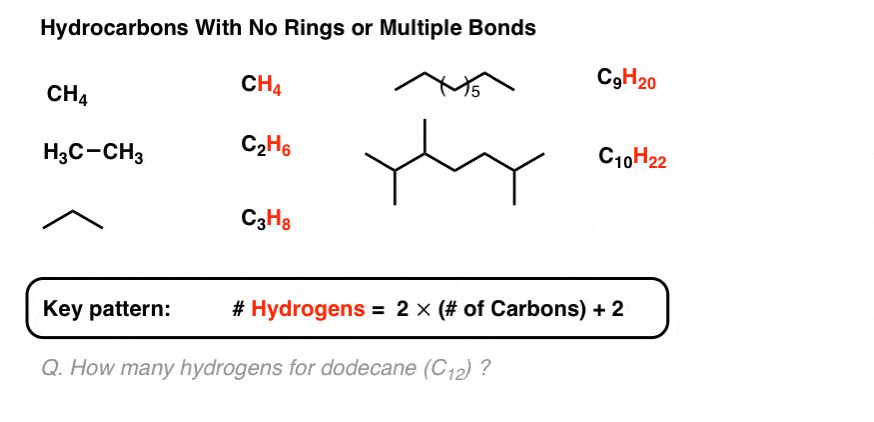
Degrees Of Unsaturation Or Ihd Index Of Hydrogen Deficiency

10 Organic Chemistry Flashcards Quizlet
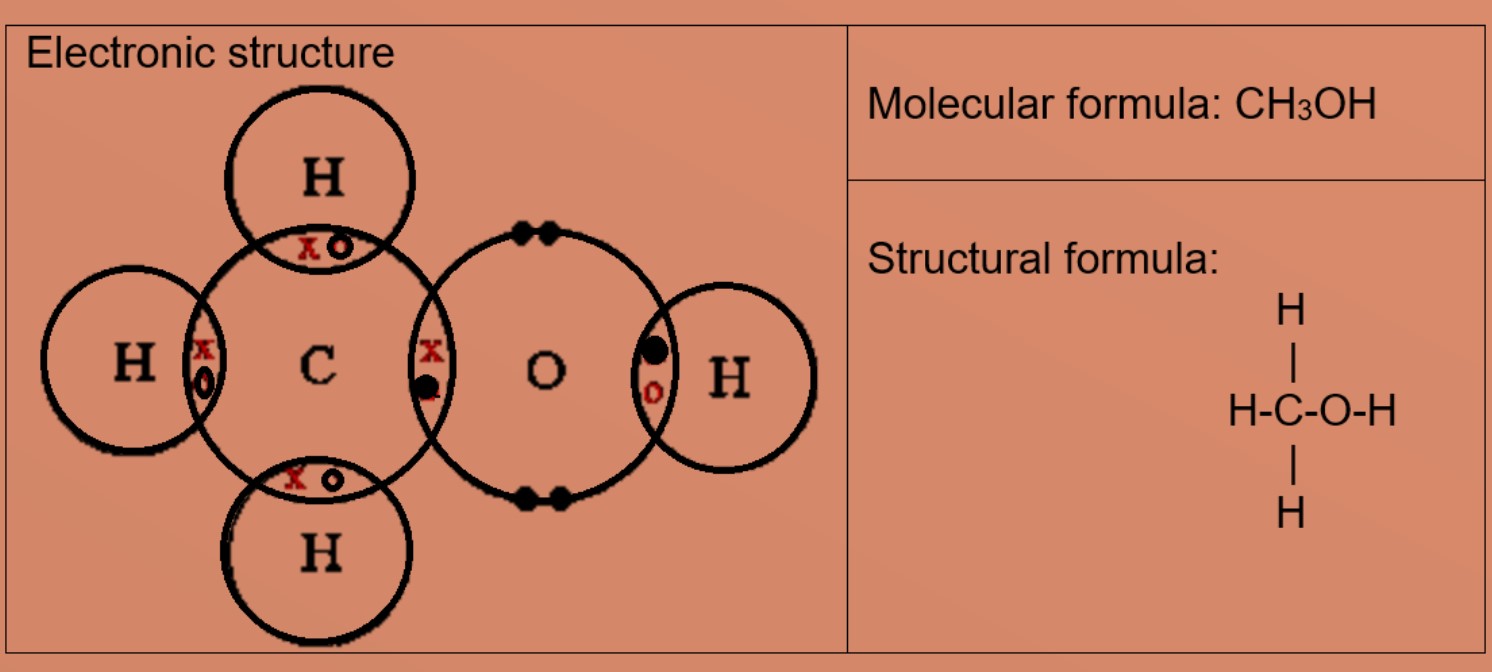
Covalent Bonding And Molecules
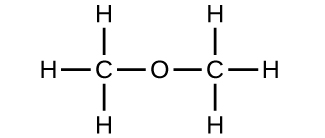
2 4 Chemical Formulas Chemistry
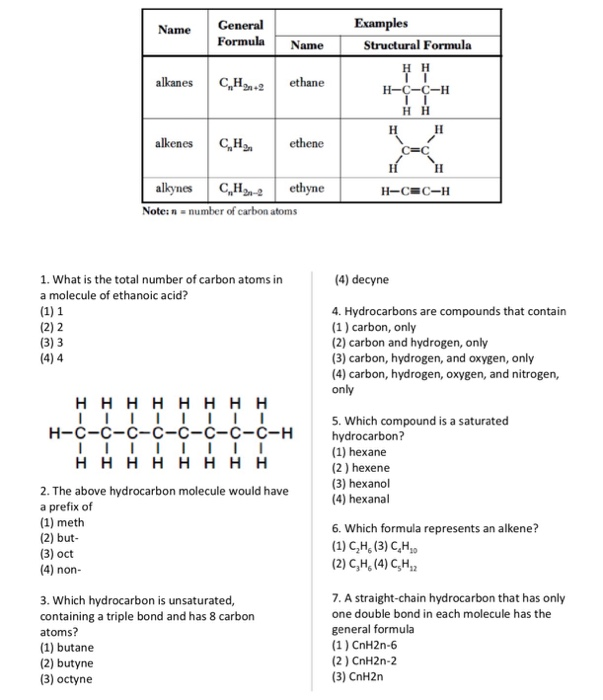
Solved Examples Name Ceneral Formula Name Structural Formula Chegg Com
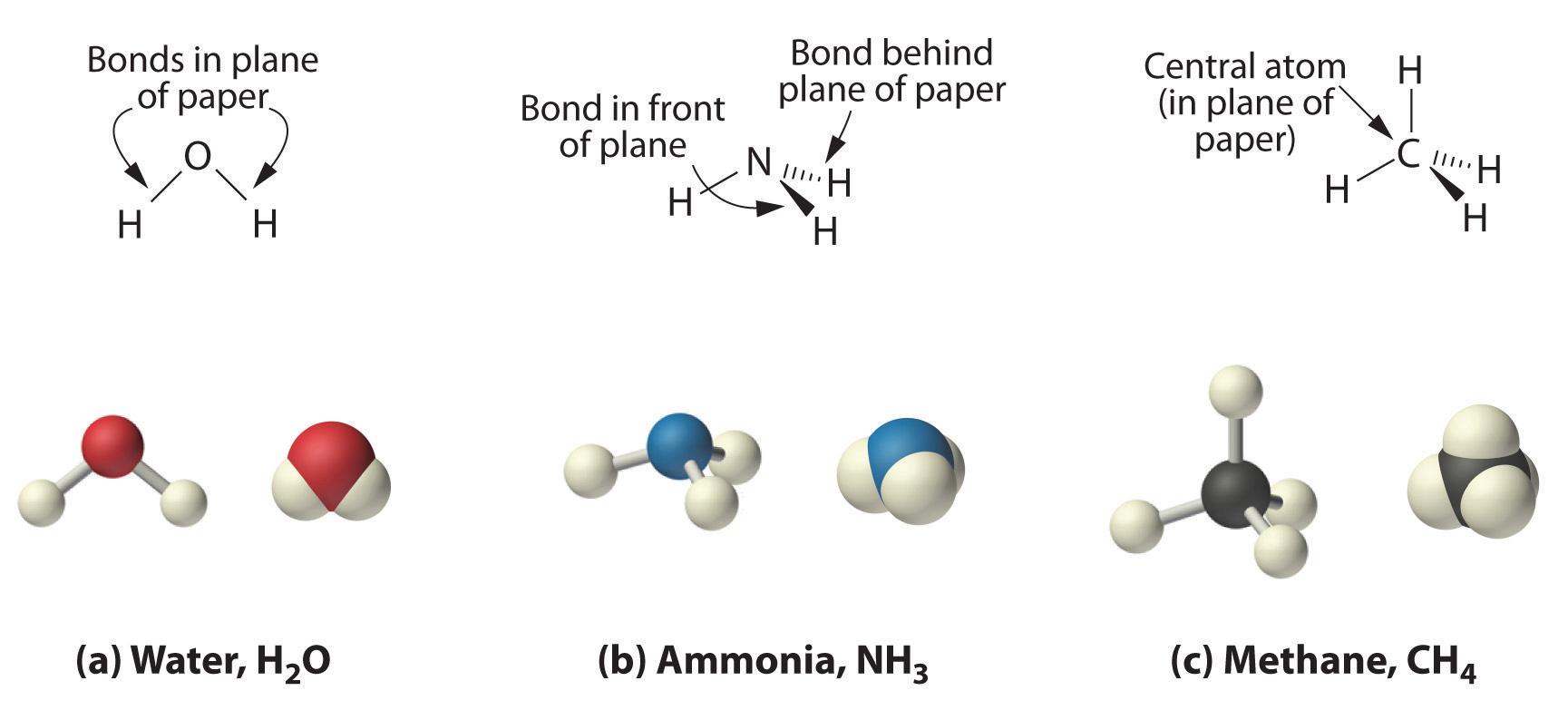
Molecules Ions And Chemical Formulas

Nitrogen Atom An Overview Sciencedirect Topics


Comments
Post a Comment